IDH Mutations
in Glioma
Evolving Current Understanding
of Gliomagenesis, Prognosis,
and Disease Classification
Evolving Current Understanding of Gliomagenesis, Prognosis, and Disease Classification
Mutations in IDH are Drivers
of Gliomagenesis
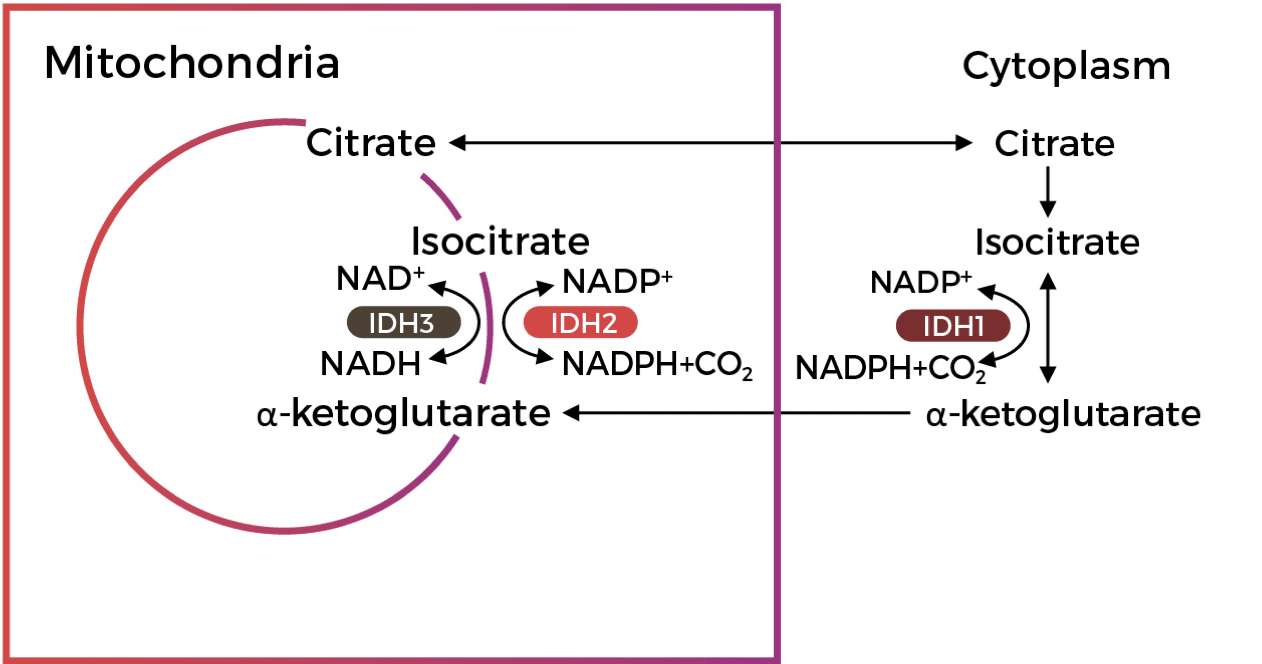
Mutations in the Metabolic Enzyme IDH
are Drivers of Gliomagenesis1,2
Mutations in the Metabolic Enzyme IDH are Drivers of Gliomagenesis1,2
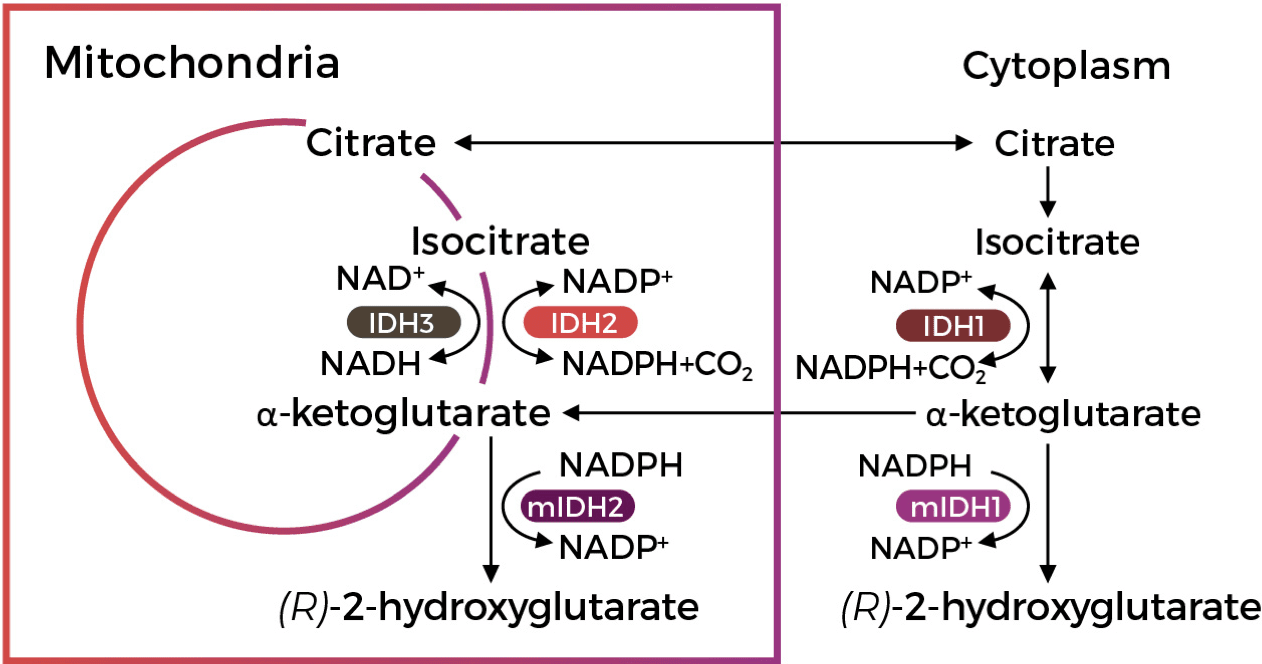
mIDH and Increased 2-HG Levels Impact
Multiple Cellular Processes Implicated in
Cancer Biology and Gliomagenesis2,3
mIDH and Increased 2-HG Levels Impact Multiple Cellular Processes Implicated in Cancer Biology and Gliomagenesis2,3
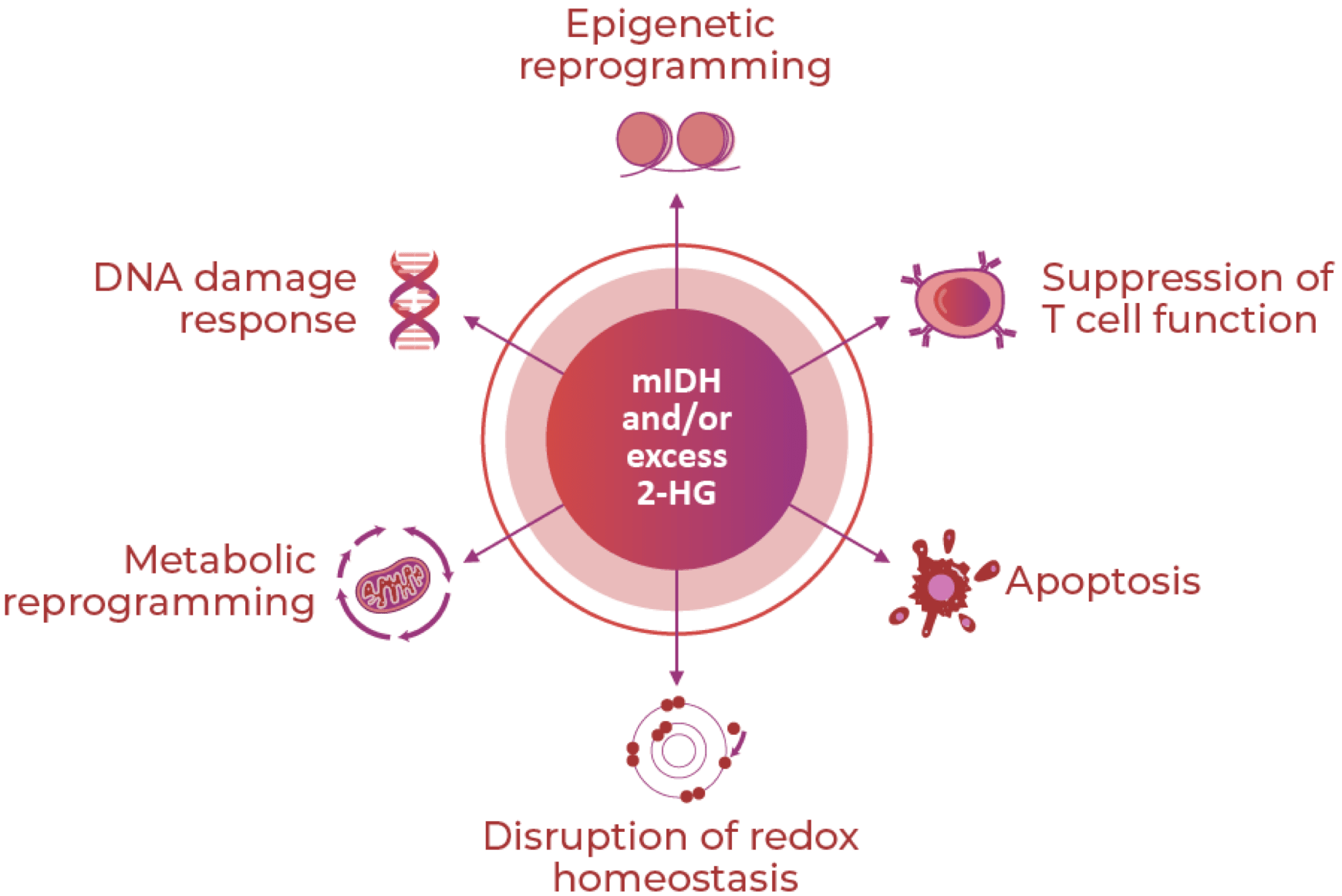
Epigenetic reprogramming
IDH mutations may impair cellular differentiation and play a role in malignant transformation of glioma by contributing to global DNA hypermethylation and histone methylation.2
Suppression of T cell function
IDH mutations may alter the tumor immune microenvironment by suppressing tumor-infiltrating lymphocytes, natural killer cells, and cytotoxic T cells.2
Apoptosis
IDH mutations may exert oncogenic effects independent of 2-HG by decreasing sensitivity to apoptosis and promoting unchecked cell proliferation.3
Disruption of redox homeostasis
IDH mutations may contribute to genomic instability and unrestricted cellular growth, motility, and invasiveness by reducing scavenging of reactive oxygen species and increasing oxidative damage.2
Metabolic reprogramming
IDH mutations confer neomorphic enzymatic activity, which may result in a unique metabolic pattern. Epigenetic silencing of the glycolytic pathway may explain the slow-growing nature of IDH-mutated glioma and increased glutaminolysis may be a compensatory pathway to maintain metabolic homeostasis.2
DNA damage response
IDH mutations may increase sensitivity to radiotherapy and chemotherapy by interfering with DNA repair enzyme activity.2
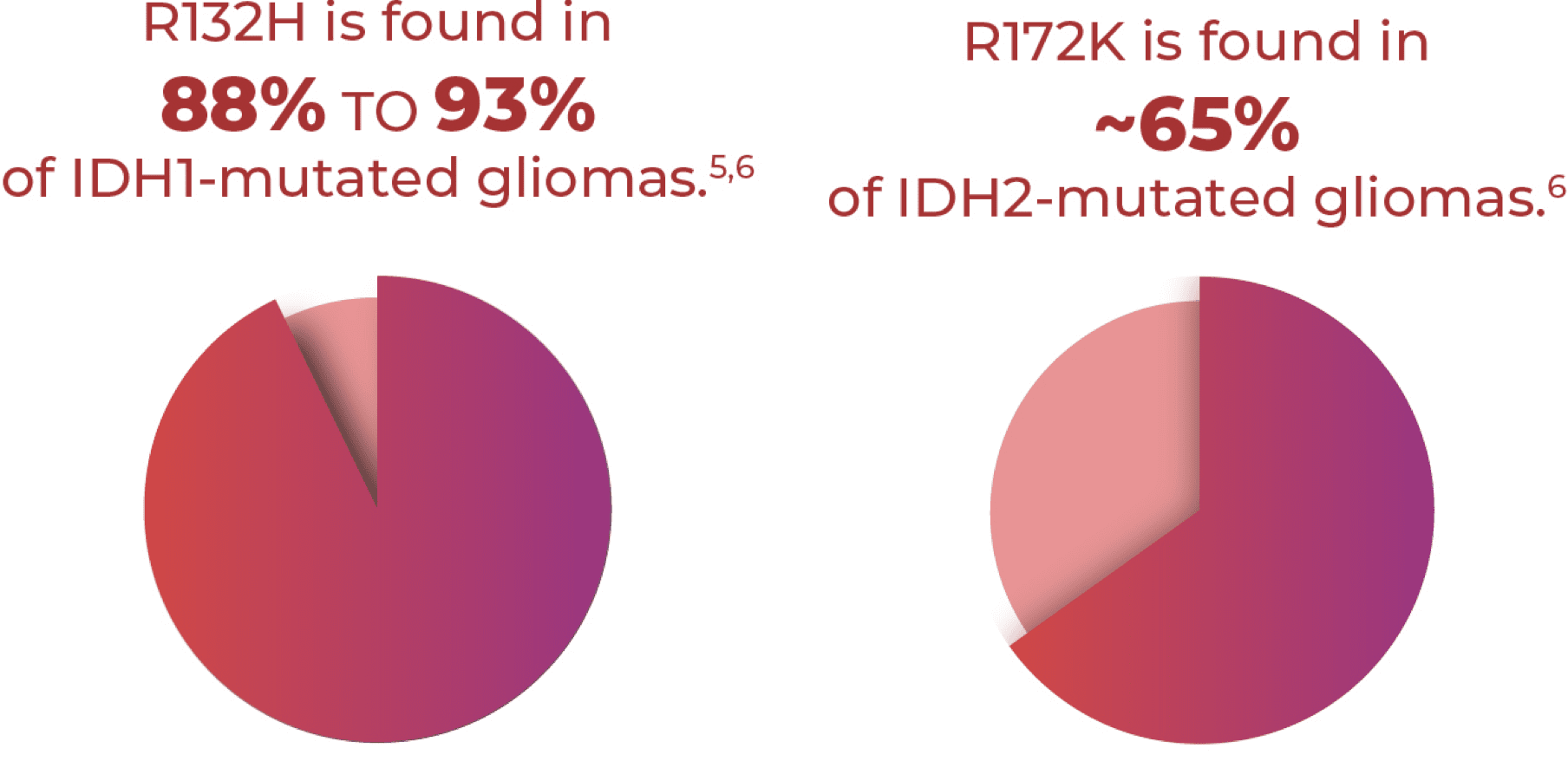
R132H and R172K are the Most Common Gain-
of-Function Point Mutations in IDH1 and IDH2
Mutated Gliomas, Respectively4-7
R132H and R172K are the Most Common Gain- of-Function Point Mutations in IDH1 and IDH2 Mutated Gliomas, Respectively4-7
Mutations in IDH are Essential
to Disease Classification
Establishing IDH Mutation Status Is Essential
to the Accurate Diagnosis and Appropriate
Treatment of Adult-Type Diffuse Gliomas8
Establishing IDH Mutation Status Is Essential to the Accurate Diagnosis and Appropriate Treatment of Adult-Type Diffuse Gliomas8
2021 WHO classification of CNS tumors
relies on key molecular features and histology, including IDH mutation status, to diagnose adult-type diffuse gliomas.8
ASCO-SNO guidelines
utilize the 2021 WHO classification, highlighting the importance of IDH mutation status in guiding treatment of adult-type diffuse gliomas.9
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)
recommend IDH mutation testing in all patients with glioma, noting IDH mutation status impacts diagnosis, prognosis, and treatment recommendations.10
WHO Classification of Gliomas

WHO Classification of Gliomas Continues to Evolve, Emphasizing Key Molecular Features, Including IDH Mutation Status

aDiagnosis of oligoastrocytoma was strongly discouraged in the 2016 classification.12
bProtoplasmic astrocytoma, fibrillary astrocytoma, and gliomatosis cerebri were deleted from the 2016 classification.12
cAdult and pediatric diffuse glioma were grouped together in the 2016 classification.12
dOligoastrocytoma and the term “anaplastic” no longer appear in the 2021 classification.8
eThe term glioblastoma, mIDH was eliminated in the 2021 classification.8
fMolecules that are definitional are listed before others; for tumor types without specific definitional changes, more commonly altered genes and molecules are listed before others.8
Mutations in IDH are a
Prognostic Molecular Biomarker
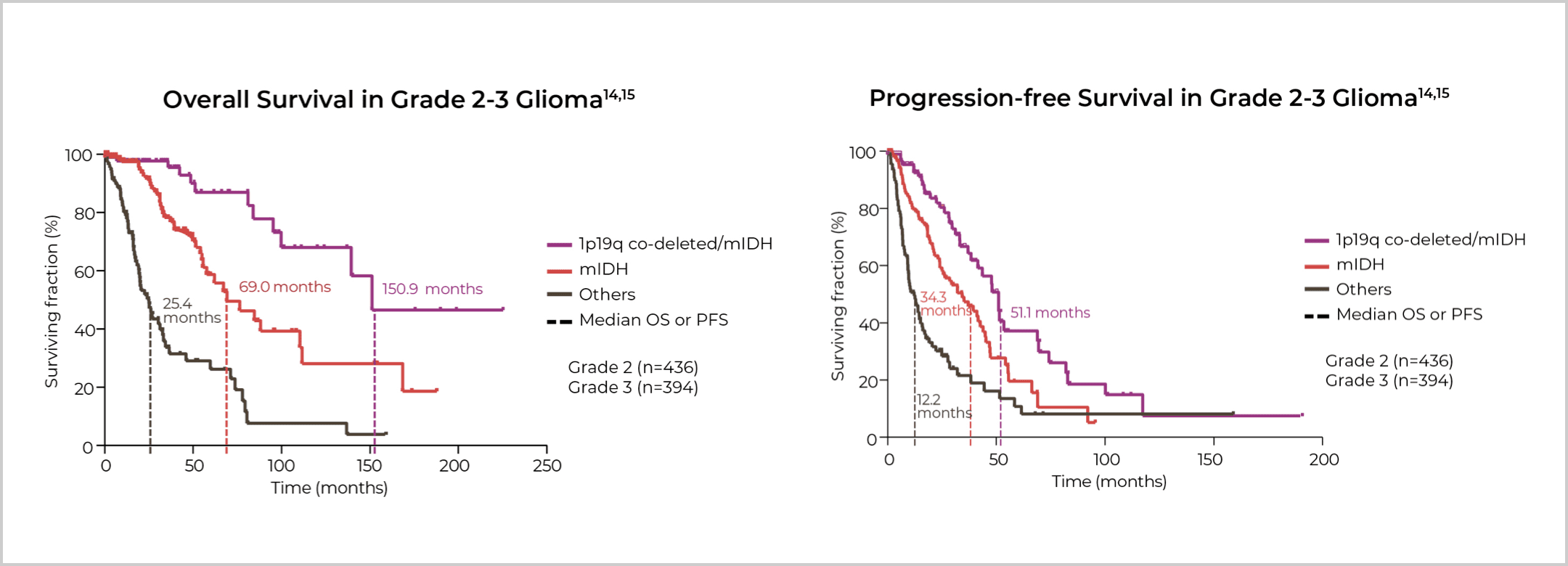
IDH Mutation Status is Prognostic in Grade
2-3 Adult-Type Diffuse Gliomas13
IDH Mutation Status is Prognostic in Grade 2-3 Adult-Type Diffuse Gliomas13
Mutations in the Metabolic Enzyme IDH are:
Drivers of
gliomagenesis2
Essential to disease
classification8
A prognostic
molecular biomarker13
Stay in the Know
Keep up to date on the latest in
IDH science from leading experts.
1p/19q, chromosomal arms 1p and 19q; 2-HG, 2-hydroxyglutarate; ASCO-SNO, American Society of Clinical Oncology-Society for Neuro-Oncology; ATRX, alpha-thalassemia mental retardation X-linked protein; CDKN2A/B, cyclin dependent kinase inhibitor 2A/B; CIC, capicua transcriptional repressor; CNS, central nervous system; CO2, carbon dioxide; DNA, deoxyribonucleic acid; FUBP1, far upstream element-binding protein 1; IDH, isocitrate dehydrogenase; mIDH, mutated isocitrate dehydrogenase; NAD, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide + hydrogen; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, nicotinamide adenine dinucleotide phosphate + hydrogen; NCCN, National Comprehensive Cancer Network; NOS, not otherwise specified; NOTCH, Neurogenic locus notch homolog protein 1; OS, overall survival; PFS, progression-free survival; R132H, arginine-to-histidine substitution at amino acid 132; R172K, arginine-to-lysine substitution at amino acid 172; TERT, telomerase reverse transcriptase, TP53, transformation-related protein 53; WHO, World Health Organization; wtIDH, wild-type isocitrate dehydrogenase.
References:
1. Losman JA, et al. Genes Dev. 2013;27(8):836-852. doi: 10.1101/gad.217406.113 2. Han S, et al. Br J Cancer. 2020;122(11):1580-1589. doi: 10.1038/s41416-020-0814-x 3. Oizel K, et al. Cell Death Dis. 2015;6(3):e1704. doi: 10.1038/cddis.2015.13 4. Miller JJ. Neurotherapeutics. 2022;19(6):1724-1732. doi: 10.1007/s13311-022-01238-3 5. Yan H, et al. N Engl J Med. 2009;360(8):765-773. doi: 10.1056/NEJMoa0808710 6. Hartmann C, et al. Acta Neuropathol. 2009;118(4):469-474. doi: 10.1007/s00401-009-0561-9 7. Tesileanu CMS, et al. Acta Neuropathol. 2021;141(6):945-957. doi: 10.1007/s00401-021-02291-6 8. Louis DN, et al. Neuro Oncol. 2021;23(8):1231-1251. doi: 10.1093/neuonc/noab106 9. Mohile NA, et al. J Clin Oncol. 2022;40(4):403-426. doi: 10.1200/JCO.21.02036 10. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Central Nervous System Cancers V.1.2024.© National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 10, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 11. Louis DN, et al. Acta Neuropathol. 2007;114(2):97-109. doi: 10.1007/s00401-007-0243-4 12. Louis DN, et al. Acta Neuropathol. 2016;131(6):803-820. doi: 10.1007/s00401-016-1545-1 13. Sharma A, et al. Ann Palliat Med. 2021;10(1):863-874. doi: 10.21037/apm-20-640 14. Murugan AK, et al. Br J Biomed Sci. 2022 Jan 31:79:10208. doi: 10.3389/bjbs.2021.10208 15. Wang XW, et al. Biomed Res Int. 2014;2014:540236. doi: 10.1155/2014/540236
